0 Terms
0 TermsHome > Terms > Serbo Croatian (SH) > Šarlov zakon
Šarlov zakon
The volume of a gas is directly proportional to its temperature in kelvins, if pressure and amount of gas remain constant. Doubling the kelvin temperature of a gas at constant pressure will double its volume. If V1 and T1 are the initial volume and temperature, the final volume and temperature ratio V2/T2 = V1/T1 if pressure and moles of gas are unchanged.
0
0
Improve it
- Part of Speech: noun
- Synonym(s):
- Blossary:
- Industry/Domain: Chemistry
- Category: General chemistry
- Company:
- Product:
- Acronym-Abbreviation:
Other Languages:
Member comments
Terms in the News
Featured Terms
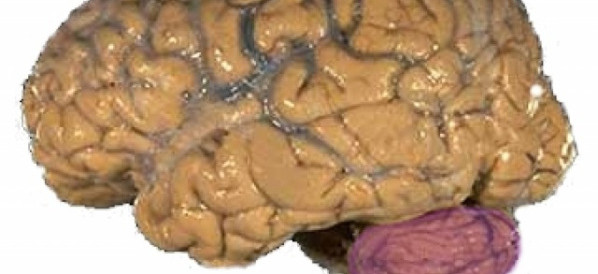
Industry/Domain: Anatomy Category: Human body
mali mozak
The portion of the brain in the back of the head between the cerebrum and the brain stem.
Contributor
Featured blossaries
Browers Terms By Category
- Physical geography(2496)
- Geography(671)
- Cities & towns(554)
- Countries & Territories(515)
- Capitals(283)
- Human geography(103)
Geography(4630) Terms
- General boating(783)
- Sailboat(137)
- Yacht(26)
Boat(946) Terms
- Algorithms & data structures(1125)
- Cryptography(11)
Computer science(1136) Terms
- General architecture(562)
- Bridges(147)
- Castles(114)
- Landscape design(94)
- Architecture contemporaine(73)
- Skyscrapers(32)
Architecture(1050) Terms
- Festivals(20)
- Religious holidays(17)
- National holidays(9)
- Observances(6)
- Unofficial holidays(6)
- International holidays(5)